The human brain, a complex organ weighing just about three pounds, is central to our cognitive functions, emotions, and overall well-being, processing vast amounts of information through approximately 86 billion neurons and trillions of synaptic connections1.
Physically made up of gray matter, which contains neuron cell bodies, and white matter, which consists of axons connecting different brain regions, the brain orchestrates the intricate processes that define human consciousness and behavior.
However, with age, the brain’s efficiency in clearing out waste diminishes, particularly due to reduced activity of the glymphatic system, and this leads to an increased risk of neurodegenerative diseases and cognitive decline. This decline in the brain’s disposal system has been linked to neurodegenerative diseases such as Alzheimer’s and Parkinson’s, often referred to as “dirty brain” diseases.
Scientists have now successfully demonstrated that a drug, already used for labor induction, can effectively restore this critical trash-clearing function, which has ignited hope for new treatments for neurodegenerative diseases that were previously hardly treatable.
The glymphatic system: brain’s waste disposal mechanism
In 2012, Maiken Nedergaard and her team first described the glymphatic system, the brain’s unique trash removal process.
This system uses cerebrospinal fluid (CSF) to wash away excess proteins and other waste products generated by neurons and other cells during normal brain activity by facilitating the flow of CSF through the brain’s extracellular space – meaning the CSF travels through a network of channels and perivascular spaces, effectively flushing out metabolic waste and ensuring a cleaner, healthier brain environment.
In young, healthy brains, the glymphatic system effectively flushes out these toxic proteins, but as we age, the system’s efficiency declines. This inefficiency sets the stage for the accumulation of harmful substances like beta-amyloid and tau proteins in Alzheimer’s, and alpha-synuclein in Parkinson’s, contributing to the onset and progression of these diseases.

The glymphatic system supports interstitial solute and fluid clearance from the brain. (A) To evaluate the role of the clearance of interstitial solutes, we measured the elimination of intrastriate [3H]mannitol from the brain. Over the first 2 hours after injection, the clearance of intrastriate [3H]mannitol from Aqp4-null mouse brains was significantly reduced (*P < 0.01, n = 4 per time point) compared to WT controls. (B) Schematic depiction of the glymphatic pathway. In this brain-wide pathway, CSF enters the brain along para-arterial routes, whereas ISF is cleared from the brain along paravenous routes. Convective bulk ISF flow between these influx and clearance routes is facilitated by AQP4-dependent astroglial water flux and drives the clearance of interstitial solutes and fluid from the brain parenchyma. From here, solutes and fluid may be dispersed into the subarachnoid CSF, enter the bloodstream across the postcapillary vasculature, or follow the walls of the draining veins to reach the cervical lymphatics. Description/Figure Credit: ncbi.nlm.nih.gov
The glymphatic system’s efficiency in fluid transport and waste clearance is profoundly dependent on the sleep-wake cycle, as research suggests. Optimal glymphatic function necessitates a well-structured sleep architecture that includes both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep stages.
Deep slow-wave sleep during NREM stage 3, characterized by slow-wave (delta wave) EEG activity, notably plays a crucial role in enhancing glymphatic flow. This stage reduces resistance within the brain’s interstitial space, thereby facilitating more effective cerebral waste removal.
Conversely, sleep deprivation impairs this process, with recovery sleep failing to fully restore glymphatic efficiency following total sleep loss.
Moreover, disruptions in sleep architecture adversely affect glymphatic function. Aging is associated with a shift towards shallower and more fragmented sleep, which impairs the efficiency of waste clearance. This decline in sleep quality is directly correlated with reduced glymphatic flow in older adults.
In addition, medications that interfere with slow-wave sleep, such as benzodiazepines, may exacerbate cognitive decline. This highlights the importance of exploring alternative treatments that promote deeper slow-wave sleep, thereby supporting glymphatic function and potentially mitigating the risk of dementia.
The role of lymph vessels in waste clearance
Research has shed light on the specific pathways through which waste-laden CSF exits the brain, particularly through the cervical lymph vessels in the neck. These vessels are crucial in transporting CSF from the brain to the lymphatic system, where it is processed and eventually eliminated from the body.
The research team at the University of Rochester, led by Douglas Kelley, used advanced imaging and particle tracking techniques to detail this route, revealing that about half of the dirty CSF exits through these vessels.
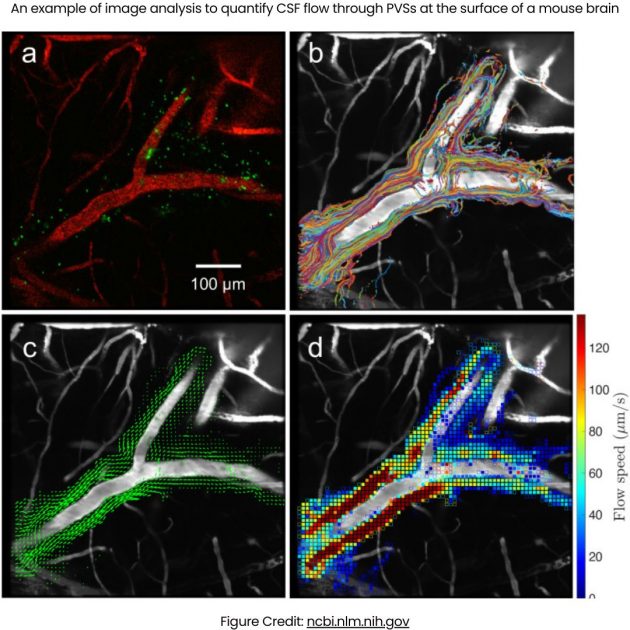
This is an illustration of how lymphatic vessels in the dura might drain to the deep cervical lymph nodes (DCLN). A: The ventral aspect of the rat skull is shown; the right side shows the brain in situ. The jugular foramen is highlighted and shows the exit of the vagal nerve (X), internal jugular vein (IJV) and internal carotid artery (ICA). B: High magnification of the area of the jugular foramen with the proposed exiting vessels and vagal nerve. Lymphatic vessels (LV) associated with dura lining the ventral surface of the skull might exit at this site into the DCLNs. This drawing is a proposed illustration of the connection between dural LVs and DCLNs.
A key finding from this study was the identification of tiny pumps, known as lymphangions, within the lymphatic system. Unlike the cardiovascular system, which relies on the heart as a central pump, the lymphatic system uses a network of these microscopic pumps to transport fluid.
However, with aging, the frequency of lymphangion contractions decreases, and the valves within them begin to fail, slowing the flow of CSF out of the brain by 63% compared to younger brains. This slowdown contributes significantly to the toxic buildup, such as beta-amyloid plaques, associated with neurodegenerative diseases.
Prostaglandin F2α: a potential therapeutic intervention
Adding to the advancement of brain science, the Rochester team has identified prostaglandin F2α (PGF2α), a compound traditionally used in labor induction, as a potential agent for restoring the brain’s waste disposal system.
PGF2α enhances smooth muscle contraction, which is crucial for lymphangion function. Applied to the cervical lymph vessels of older mice, PGF2α significantly increased contraction frequency and CSF flow, restoring waste-clearing efficiency to levels observed in younger mice. This discovery highlights PGF2α’s potential beyond its conventional use and suggests a novel approach to managing age-related neurodegeneration.

Mechanical engineering professor Douglas Kelley (left) and assistant professor Ting Du from the University of Rochester Medical Center’s Department of Neurology examine how cervical lymphatic vessels drain cerebrospinal fluid from the brain. Changes to that flow as we age increase the risk of Alzheimer’s, Parkinson’s, and other neurological disorders. Description/Photo Credit: (University of Rochester photo / J. Adam Fenster)
Building on these findings, the researchers administered PGF2α to aged mice and assessed its impact on lymphatic contractions and CSF dynamics. Their thorough review of PGF2α’s effects on smooth muscle and lymphatic function guided their hypothesis.
The results demonstrated a remarkable improvement in both contraction frequency and CSF flow, indicating that PGF2α could be repurposed for treating or preventing age-related neurodegenerative disorders. This evidence supports the idea that PGF2α may play a role in enhancing brain waste clearance, which is critical for addressing diseases like Alzheimer’s and Parkinson’s.
The proximity of lymph vessels to the skin presents a promising opportunity for non-invasive treatments. This approach aligns with the growing trend towards personalized medicine and could lead to the development of topical formulations or patches containing PGF2α.
By repurposing existing drugs like PGF2α, researchers could streamline drug development, reduce costs, and accelerate the availability of treatments. Such innovations could improve the management of neurodegenerative conditions, potentially extending healthy cognitive function and making treatments more accessible.
Broader impact On cognitive health
Enhancing the brain’s waste disposal system extends far beyond merely clearing toxic proteins. Neurodegenerative diseases like Alzheimer’s and Parkinson’s involve progressive neuron loss and cognitive decline due to protein waste accumulation.
It may be possible to slow or halt the progression of these diseases by by improving the efficiency of the glymphatic system through interventions like PGF2α. This broader impact underscores the significance of effective waste clearance mechanisms in maintaining cognitive health.
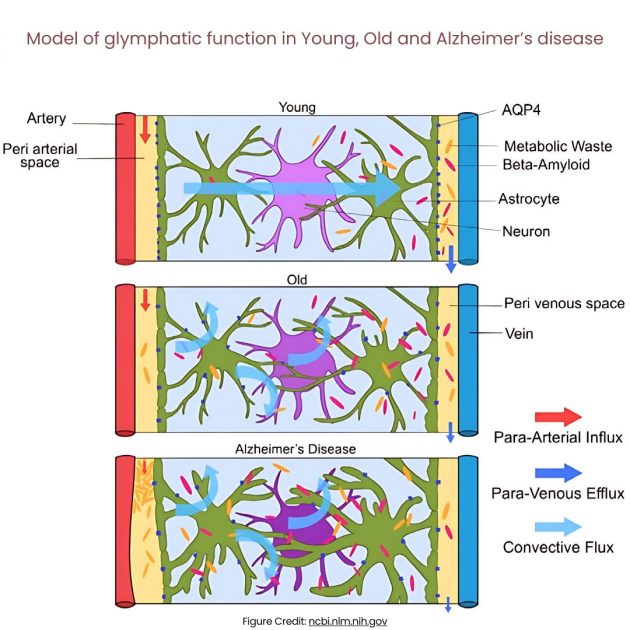
Figure illustrated by Jessamyn Camille Reddy. Model of glymphatic function in Young, Old and Alzheimer’s disease. In young people, CSF travels along periarterial routes, entering the brain parenchyma, and washes solutes and waste products into the veins. In older people, the loss of AQP4 water channels will result in reduced glymphatic clearance. In those with Alzheimer’s disease, the accumulation of amyloid-beta impairs fluid movement within the interstitial space, decreasing glymphatic clearance.
Cognitive decline is common in both Alzheimer’s and Parkinson’s, though significantly less common in Parkinson’s, as James M. Ellison, MD, MPH of the Swank Center for Memory Care and Geriatric Consultation, ChristianaCare, writes. Many as half of people with Parkinson’s, according to Ellison, develop cognitive difficulties, ranging from mild forgetfulness to full-blown dementia.
In this context, research has also demonstrated that the speed at which damaged proteins are cleared from neurons is critical for cell survival. In Huntington’s Disease, for instance, faster clearance of the mutant huntingtin protein is associated with prolonged neuronal survival.
This finding supports the notion that enhancing proteostasis – the process by which cells regulate protein levels and quality – could be a key strategy in combating neurodegenerative diseases. Improved proteostasis and waste clearance mechanisms are essential for developing effective treatments and managing disease progression.
The significance of proteostasis and autophagy
Proteostasis’s role is vital in maintaining the health of neurons by ensuring that proteins are properly folded and that damaged or misfolded proteins are quickly degraded. The study by Tsvetkov and colleagues showed that differences in the rate of proteostasis might explain why certain neurons are more susceptible to death in neurodegenerative diseases like Huntington’s.
One of the key mechanisms for protein degradation is autophagy2, where cells break down and recycle damaged proteins. The research indicated that neurons increase autophagy rates in response to the accumulation of mutant huntingtin, highlighting autophagy as a potential drug target for neurodegenerative diseases. Enhancing autophagy could help to improve the clearance of toxic proteins, thereby protecting neurons from death and preserving brain function.
Challenges and future directions
While these findings are promising, several challenges remain before such treatments can be widely applied to humans.
The transition from mouse models to human patients is complex, especially because the physiological differences between species can lead to variations in drug metabolism, immune responses, and overall treatment outcomes, and further research is needed to determine the safety and efficacy of PGF2αv – as its affects on preterm birth and related complications have shown variability – and other potential interventions in humans.
Furthermore, understanding why the brain’s coping mechanisms fail with age, as scientifically evidenced by the decline in neuroplasticity and synaptic density, is crucial for developing effective treatments.
Based on this, future research will require to focus on identifying other drugs or interventions that can enhance the glymphatic system and proteostasis in aging brains, according to the Rochester researchers emphasizing their crucial role in brain health. Moreover, new research methods are essential not only for deepening our understanding of individual neuron functionality and their response to therapeutic interventions but also for assessing these interventions at a more detailed level.
These methods will be essential in advancing personalized treatments for neurodegenerative diseases; however, the primary drawback, as noted by the researchers, is the significant cost and complexity involved in applying these sophisticated techniques in clinical practice.
Footnotes:
- In the human brain, some 86 billion neurons form 100 trillion connections to each other – numbers that, ironically, are far too large for the human brain to fathom. ↩︎
- Autophagy is a cellular degradation and recycling process that is highly conserved in all eukaryotes. In mammalian cells, there are three primary types of autophagy: microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA). While each is morphologically distinct, all three culminate in the delivery of cargo to the lysosome for degradation and recycling. During microautophagy, invaginations or protrusions of the lysosomal membrane are used to capture cargo. Uptake occurs directly at the limiting membrane of the lysosome, and can include intact organelles. ↩︎
- Can LLMs generate better research ideas than humans? A critical analysis of creativity and feasibility - September 25, 2024
- Artificial Super Intelligence: Transcending Imagination - September 15, 2024
- Artificial General Intelligence: Start of a New Era - September 8, 2024



